Medical Devices Conformity Assessment
Why Need Medical Devices Conformity Assessment?
Section 10 of Medical Device Act ( Act 737 ) requires a medical device to undergo conformity assessment by a registered CAB prior to its registration. However, many medical devices have undergone conformity assessment and approved in countries recognized by Medical Device Authority (MDA).
MDA Circular Letter No 2/2014 sets the policy relating to conformity assessment for medical devices approved by countries recognized by MDA. The policy simplifies the process of conformity assessment and accelerate medical device registration under Act 737.
All medical devices manufactured, imported or distributed in Malaysia shall be subjected to conformity assessment as stipulated as per above requirements.
Medical devices which have not been obtained with any approvals by regulatory authorities or notified bodies listed in Table 1 are required to undergo full conformity assessment by any registered CAB in accordance with the requirements as stipulated in Section 7(1)(a) of Act 737.
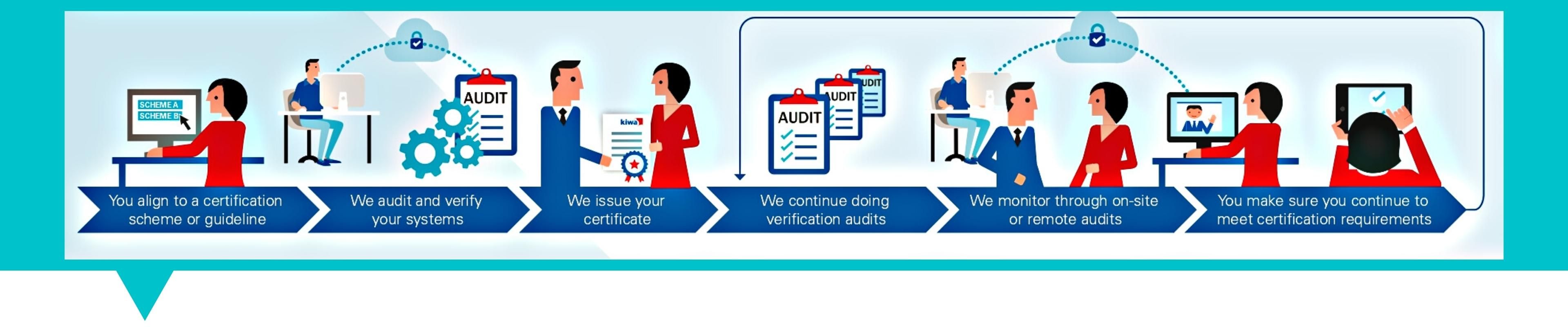
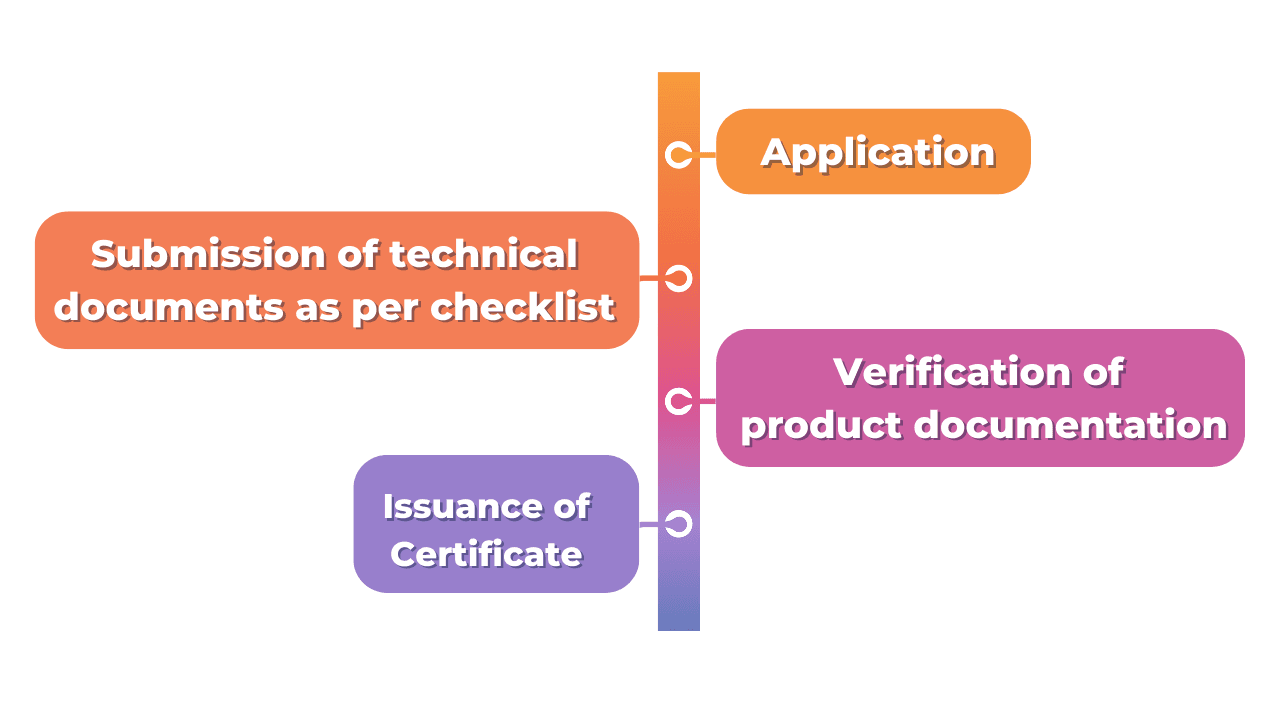
The Certification Process
Let us answer you
Frequently Asked Questions

Get Free Quote
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05
Kiwa International Certifications(M) Sdn Bhd
2A, Jalan Astana 1D, Bandar Bukit Raja, 41050 Klang, Selangor
+603-3884 7813
info@kiwacert.com
Services
Accredited to ISO/IC 17021:2015
ACB QMS 28
ACB OSH 17
ACB EMS 20
ACB GMP 06
ACB FSMS 13
ACB HACCP 08
ACB MDMS 05